Contents
1. Introduction
1.1 This guidance is produced on the best available understanding of the issues; however, organisations that provide care and support should also refer to the latest CQC guidance, and if necessary, their own legal advice, in more complex matters.
1.2 For those adults that need to take medication to maintain their health and wellbeing, it is essential to ensure that the adult has the right level of medication and has access to medication when necessary.
1.3 Wherever possible the adult should be encouraged to discuss, with appropriate support, any medication issues they are experiencing with their prescriber or dispensing pharmacist.
1.4 It is also important that medication is not given without consent. Medication needs to be administered in accordance with the Mental Capacity Act (2005) and Mental Health Act (1983).
1.5 If the adult is unable to consent, then medication needs to be administered in accordance with the principles of the Mental Capacity Act. These must be reflected in the care plan and the care plan should be followed and regularly reviewed.
1.6 In the event of a medication error advice should always be sought from the prescriber or dispensing pharmacist. This document explains that this is important because some medicines can cause more harm than others when omitted, delayed or overdosed.
1.7 All guidance provided with the medication should be read before it is administered.
1.8 Advice should be sought from the dispensing pharmacists or the NHS Devon Guidance Sheets before a medication that does not include an instruction to take with food is mixed with food.
1.9 Many safeguarding adult concerns are raised to the Torbay and South Devon NHS Foundation Trust and Devon County Council concerning medication errors. This guidance has been produced to:
- Assist those organisations who provide care and support services to determine when they should raise a safeguarding adult concern to Torbay and South Devon NHS Foundation Trust or Devon County Council safeguarding adult teams; and,
- Ensure that those organisations who provide care and support services are aware of when a safeguarding adult concern should be raised to the Torbay and South Devon NHS Foundation Trust and Devon County Council safeguarding adult teams as well as the requirements for statutory notifications to the Care Quality Commission (CQC) for NHS and Non NHS providers (in respect of providers registered under the Health and Social Care Act 2008); and,
- Identify examples of the actions to be taken in respect of medication incidents that do not require a statutory notification and how these actions will be assessed by the Torbay and South Devon NHS Foundation Trust and Devon County Council safeguarding adult teams.
2. Scope
2.1 This guidance is relevant to all providers of health and social care services to adults in Torbay and Devon to enable organisations to understand when a safeguarding adult concern to Torbay and South Devon NHS Foundation Trust and Devon County Council is required. This guidance is in addition to, and does not preclude all organisations from adherence to, their own specific policies and guidance relating to medicines management and incident management, along with any other relevant legislation, policy and guidance.
2.2 Where the error has taken place in a hospital, if following NHS internal processes there remains doubt as to whether a safeguarding referral should be made, then guidance must always be sought from the Safeguarding Adult lead within the hospital and/or the Torbay and South Devon NHS Foundation Trust and Devon County Council Safeguarding Service.
2.3 Where controlled medicines are involved this should be referred to the police if there is a concern that a crime has been committed.
3. When should a medication error be raised as a safeguarding adult concern to Torbay and South Devon NHS Foundaton Trust or Devon County Council?
3.1. A Safeguarding adult concern referral will always need to be raised where the medication error triggers a notification to CQC and/or a report to the Police.
3.2. A CQC notification is required where the cause or effect of a medication error results in:
- Death
- Injury
- Abuse or an allegation of abuse
- An incident reported to or investigated by the police
3.3 In addition, Torbay and South Devon NHS Foundation Trust and Devon County Council expects a safeguarding adult concern to be raised where the person or persons in question came to harm and/or required a hospital admission.
In this context ‘harm’ is defined as any physical or mental change experienced by an individual that is caused by, or considered likely to have been caused by, the error which results in a permanent increase to a person’s care and support needs and/or a high level of distress.
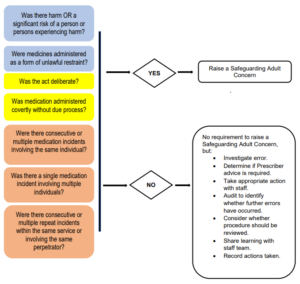
3.4 If any of the following occur, a Safeguarding adult concern MUST also be raised:
- Medication is given as a form of unlawful restraint (e.g., a non-prescribed sedative is administered, or a prescribed medicine is administered at a higher dose or more frequently than prescribed).
- A deliberate act to administer/neglect to administer medication contrary to the directions of the prescriber (e.g., deliberately increasing the dose of a medication or failing to administer it).
- A medication is administered covertly (see Appendix 2 for more information about covert administration) where no specific approved covert medication protocol is in place (e.g., administering a tablet in yoghurt where an Individual with or without capacity has refused).
- Consecutive/multiple medication incidents involving the same individual (e.g., prescribed medication is not administered over more than one round because it has not been ordered or collected) and the person experiences harm.
- Single medication incident involving multiple individuals (e.g., a whole medication round missed or delayed) and any one person experiences harm.
- Multiple/repeat incidents within the same service, or by the same perpetrator (e.g., medication is administered incorrectly by a specific member of staff on more than one occasion).
- Where a controlled medicine is involved and there has been harm and/or a crime.
- Where a referral is required to a professional body, for example Nursing & Midwifery Council (NMC)
4. What action is required if a medication error does NOT trigger raising a Safeguarding adult concern?
4.1 Whether or not a medication error triggers raising a Safeguarding adult concern, any identified poor practice in administration of medication requires a management response.
4.2 This is because poor practice at any level which is not addressed can lead to medication errors which have a negative impact on individuals. Acting in response to all medication errors mitigates against the risk of reoccurrence and improves practice. Actions include:
Audit – Conducting a robust, regular audit of medication systems will assist in ensuring that errors and trends are quickly identified. Look out especially for medications which sometimes have variable doses (e.g., Warfarin) those which are non-routine (e.g., antibiotics) and those stored other than in the medication cabinet (e.g., eyedrops and some topical creams) as errors often occur with these. Refrigerated medication must be stored according to manufacturer’s guidelines.
A good audit will check that stock is ordered in good time, that medication from the pharmacy is confirmed correct on receipt, that recording of administration, refusal and disposal is accurate, expiry dates are reviewed and that recording of administration is consistent with stock held. The frequency of audit should be increased where new staff are deployed to administer medication, and in response to errors identified.
Training and guidance should be sought on best practice, and this should be regularly reviewed and updated.
Investigate – It is important to investigate the root cause of any medication error to determine whether written procedures need to be reviewed, individuals or teams of staff require additional training, or whether the risk of accidental error can be mitigated by implementing changes to practice.
For more widespread concerns a thorough investigation report will capture detail which might include statements of involved staff, anonymised copies of MAR sheets, care delivery records, communication with relevant parties (e.g. GP, Safeguarding, CQC), a written factual account of the investigation conducted, conclusion and action taken.
Record – Both as an audit tool and to evidence the action you’ve taken it is necessary to maintain a record of medication errors, their investigation and the action taken to address the incident. Organisations are required to put in place their own documentation and systems to periodically audit medication errors to determine error trends which in turn may identify a specific training need or the requirement to raise a Safeguarding Adult concern. Local Authorities Quality Assurance Teams and any other relevant commissioners or the CQC may request information relating to medication errors to ensure that management processes are robust.
Share learning – Even if the medication error is relatively minor in nature it is good practice to share learning across the organisation. Effectively communicating learning from investigation of medication errors is critical to creating a culture where it is acknowledged that errors can and do happen. Learning shared in a manner which promotes improved practice rather than encourages staff to hide or disguise genuine errors for fear of punishment, is likely to result in more transparent disclosure of errors where they occur.
5. Examples of poor practice which do NOT trigger a safeguarding notification:
While the examples below may not trigger a safeguarding adult concern referral, they MUST trigger a management response through training, supervision or auditing and may still be notifiable. Remember to always record what action you have taken and where the adult has capacity that they have been informed of the error, for example, where medication has not been given or given late.
A gap in recording: A signature is missed on the MAR chart, but your investigation concludes that the medicine was correctly administered, and no harm has occurred, you have taken appropriate action with the member of staff concerned and recorded this.
Medication is not given on one occasion: The adult does not receive prescribed medication (missed/wrong dose) on one occasion, advice has been sought from the prescriber and no harm occurs. You have clearly recorded the incident and taken appropriate action with the member of staff concerned.
Medication is not given on more than one occasion: Advice has been sought from the prescriber and no harm has occurred (e.g., recurring missed medication or administration errors identified through observation or audit). You take swift action once identified through training/supervision. You monitor the situation closely until poor practice has been corrected. You have clearly recorded the incident and action taken/advice given.
Medication was given late: An unforeseen event meant that some people received their medication later than scheduled, you have checked to ensure that no medication was time-sensitive and where appropriate this has been confirmed with the prescriber who has advised that no harm has occurred. You have clearly recorded the incident and action taken.
A member of staff signs the MAR chart in incorrect coloured ink; You have reminded the staff member of your policy and ensured a supply of black pens is available and removed the incorrectly coloured pens.
The pharmacy now delivers medication in patient packs instead of blister packs: You have checked that your medication procedure reflects the change in packaging, have familiarised staff with the procedure and have introduced a more frequent random ‘spot-check’ audit until you are content that the new system is working effectively. You have recorded your actions
A member of staff has changed initials and the sample signature sheet reflects their previous name: You have ensured that the member of staff has signed the sample signature sheet again with their new initials and the date on which they started to sign MAR sheets with their new initials, the original entry remains on the sample signature sheet so that previous MAR entries can be traced to this person. You have recorded your actions.
6. Examples which MAY trigger raising a safeguarding concern and where advise should be sought
- Previous concerns identified and corrective action is not maintained
- Insufficient prevention measures in place such as training, supervision, and auditing
7. Raising a safeguarding adult concern
In the first instance the organisations’ Responsible Person or Safeguarding Lead should be consulted. Should it be determined that a safeguarding adult concern referral is required then this should be made to the relevant Safeguarding Adult Service:
We encourage Safeguarding Adult concerns referrals to be raised via the Torbay and Devon Safeguarding Adults Partnership website Home – Torbay and Devon Safeguarding Adults Partnership
Alternatively, contact can be made via telephone or email as follows:
For those adults living in Torbay please call the number below or email:
01803 219700
safeguarding.alertstct@nhs.net
For those adults living in Devon please call the number below or email:
0345 155 1007
Email: adultsc.safeguardingadultservices-mailbox@devon.gov.uk
Appendix 1: Quick Reference Medication Error Decision Maker
(Any yes answer requires a concern to be raised. Any no answer refer to the 2nd box)
Appendix 2: Covert Administration of Medication
Covert administration is when medicines are administered in a disguised format.
The medicines could be hidden in food, drink or through a feeding tube without the knowledge or consent of the person receiving them. As a result, the person is unknowingly taking a medicine. Every person has the right to refuse their medicine, even if that refusal appears ill-judged to staff who are caring for them. Covert administration is only likely to be necessary or appropriate where:
- a person actively refused their medicine
- that person is judged not to have the capacity to understand the consequences of their refusal. Such capacity is determined by the Mental Capacity Act 2005
- the medicine is deemed essential to the persons health and wellbeing
Covert administration of medicines should be a last resort. You must make reasonable efforts to give medicines in the normal manner. You should also consider alternative methods of administration. This could include, for example, liquid rather than solid forms.
In circumstances where the medication is put into food to make it more palatable, and the person is aware and has agreed then it is not being administered covertly. In these circumstances their agreement should be documented.
Alway remember that administering medicines in food or drink can alter their therapeutic properties and effects. They could become unsuitable or ineffective. Always take advise from a healthcare professional to make sure medicines are safe and effective.
Further guidance on the covert administration of medicines is available from the CQC here
Acknowledgements
With thanks to Norfolk Safeguarding Adults Board and Somerset Safeguarding Adults Board whose guidance for providers has been adopted to produce this document.